
Process and background information
The Tenifer®-QPQ process is based on salt bath nitrocarburising, which involves the diffusion of nitrogen and carbon into the workpiece surface. The process produces a compound layer formed of iron, nitrogen, carbon, and nitrides, which is known for its high wear resistance and low coefficients of friction.
The process comprises four steps:
- Nitrocarburising
- Oxidative quenching
- Mechanical smoothing
- Post-oxidation
This targeted treatment maximises corrosion protection and improves the mechanical stability of the components. Since this procedure is carried out at comparatively low temperatures, changes in size and shape are kept to a minimum. Better yet, it also frequently or completely eliminates the need for costly rework.
The procedures we offer
We offer the following variants of the Tenifer® process:
- Tenifer®-Q: Basic protection against wear, corrosion, and edge-cracking problems, plus heat resistance and durability.
- Tenifer®-QP: Improved surface quality thanks to reduced roughness, lower coefficients of friction, and optimised visual quality of the components.
- Tenifer®-QPQ: Maximum corrosion resistance, decorative black surface, minimum light reflection and superior visual quality.
- Optionale KühlverfahrenThe treatments can be performed by means of water quenching or vacuum gas quenching.
The advantages at a glance
The Tenifer®-QPQ process provides a large number of technical advantages:
- High wear resistance and increased fatigue strength
- Excellent corrosion protection thanks to a layer of magnetite
- Minimum changes in dimensions and shape
- Optimum sliding performance due to the non-metallic properties of the compound layer
- Excellent mechanical resilience

The combination of edge layer hardening and oxidising treatment increases the fatigue strength of the material by up to 100%. At the same time, the treated surface reduces the frictional resistance, resulting in considerably reduced wear, especially in slide and roller bearing applications.
Applications and suitable materials
The Tenifer®-QPQ process is widely used in various industrial sectors:
- Automotive industry (e.g. gearbox components, crankshafts, gears)
- Mechanical engineering (machine parts subject to high loads)
- Aerospace (specialised precision components)
- Medical technology (instruments and components with high standards of hygiene)
- Replacement of coatings containing chrome (VI)
The treatment is suitable for almost all grades of steel and cast materials. Particularly advantageous results are achieved in materials with nitride-forming elements such as chromium, molybdenum, vanadium, and aluminium, as these make enhanced surface hardness possible.
While non-alloy and low-alloy steels achieve the highest level of corrosion protection, the treatment of stainless steels requires special modifications to the process, in order to ensure both optimum hardness characteristics and enhanced corrosion resistance.
Technical requirements for the treatment
To produce optimum results, the Tenifer®-QPQ treatment must be performed under specific process parameters. Besides the exact material designation, it is necessary to specify the temperature of the previous heat treatment, the desired hardness and the desired nitriding hardness depth. If partial treatment is desired, then appropriate drawings are necessary. In addition, to obtain the specified core hardness, the temperature of the previous heat treatment must be at least 20 to 30 degrees Celsius higher than the nitriding temperature.
Conclusion
The Tenifer®-QPQ process combines high wear resistance with excellent corrosion protection and minimum dimensional changes to provide an efficient and environmentally friendly solution for the metalworking industry.
Providing a high-performance alternative to conventional coating procedures, the process is established around the world and suitable for industrial series production, as well as for precision parts subjected to heavy loads. Thanks to its high reproducibility and ease of implementation, it represents a solution that reliably produces durable and resistant components.
Process locations
You will find Härtha in Germany, Italy, and the Netherlands. Refer to our interactive location overview to learn which other processes besides oxidising we offer at locations near you.
Der Beitrag Tenifer-QPQ erschien zuerst auf HÄRTHA GROUP.
]]>ALDOX - nitrocarburising with post-oxidation
ALDOX offers exceptionally high corrosion resistance and gives your workpieces a refined surface ranging from anthracite to black. It is an environmentally friendly alternative to the usual corrosion protection processes such as nickel plating, chrome plating or salt bath nitriding.
We use ALDOX for components that need to satisfy the most demanding technical requirements - whether as a single part, in custom sizes or as batch production parts. We are happy to be at your disposal for a personal, no-obligation consultation that will help us meet your individual requirements in the best possible way.
ALDOX-S in detail
Der Prozessablauf beim ALDOX-S-Verfahren ist nahezu identisch mit dem NIOX-Verfahren. Wir haben aber Parameter wie Temperatur, Gaszusammensetzung und Schichtaufbau angepasst, um im Salzsprühnebeltest ein optimales Ergebnis zu erzielen. So wird zum Beispiel nach dem Nitrieren die Temperatur auf Oxidiertemperatur abgesenkt.
Auf diese Weise entsteht an der Bauteiloberfläche eine 0,5 bis 2 μm dicke, dichte Oxidschicht aus Eisenoxid Fe3O4. Die Kombination aus der Nitrierschicht (Verbindungsschicht) und Oxidschicht bestimmt maßgeblich die Verbesserung der Korrosionsbeständigkeit.
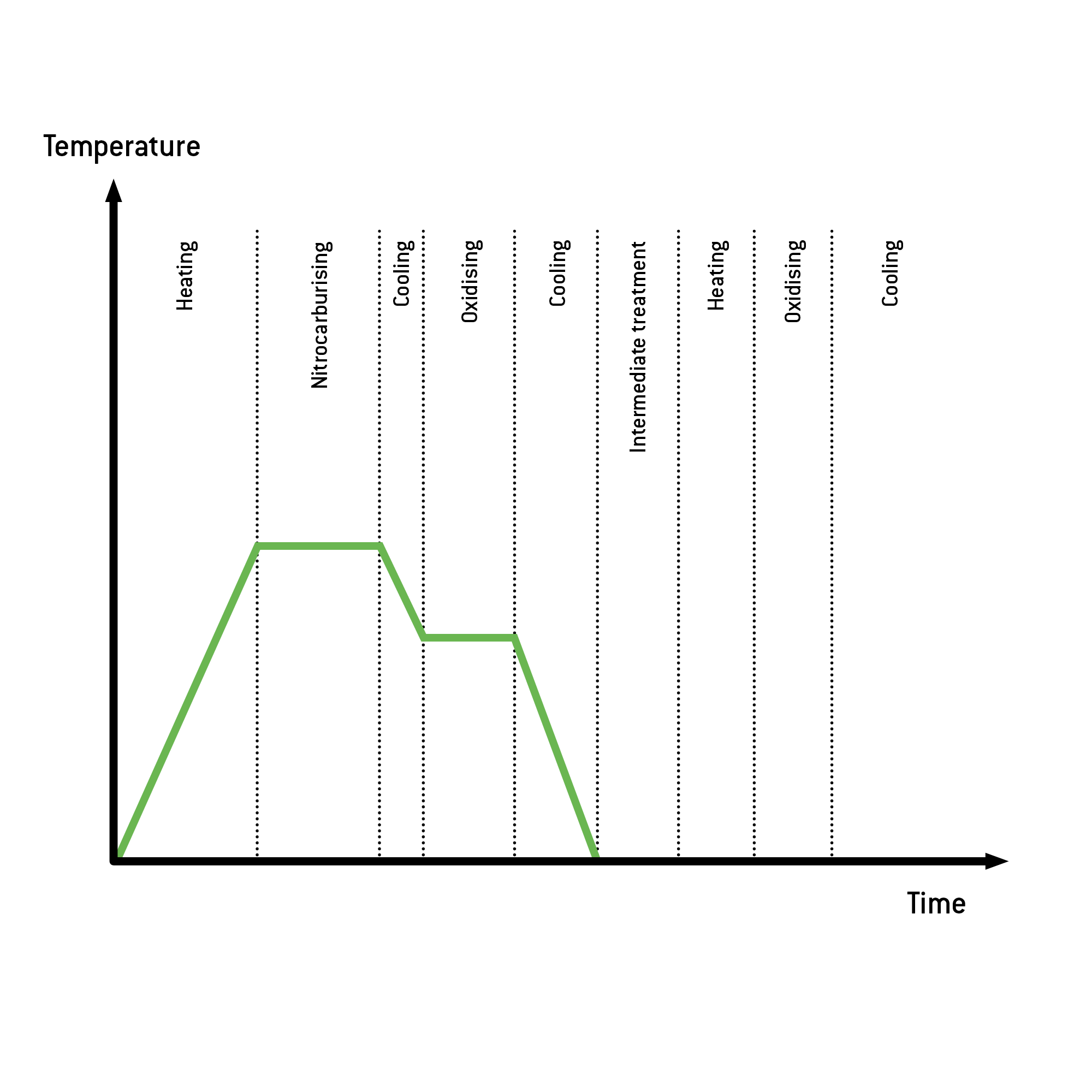
Process flow ALDOX-S
ALDOX-P in detail
ALDOX-P is distinguished from ALDOX-S by the addition of an intermediate treatment and another oxidation process. This results in the creation of a component surface with anoxide layer composed of iron oxide Fe3O4 that is 1 to 3 µm thick and offers excellent adhesion.The combination of the nitrided layer, acting as a compound layer, with this oxide layer leads to significantly improved corrosion resistance in the treated workpiece.
Intermediate treatment & additional oxidation process:
The optimized nitrocarburising is followed up with a post-oxidation of the workpieces . In this step, the compound layer produced in the preceding step is partially converted into an oxide layer by dwelling and cooling in an oxidising environment. The process concludes with another complete oxidation process (heating, oxidising, and cooling). This lends an additional oxide layer to the workpieces.
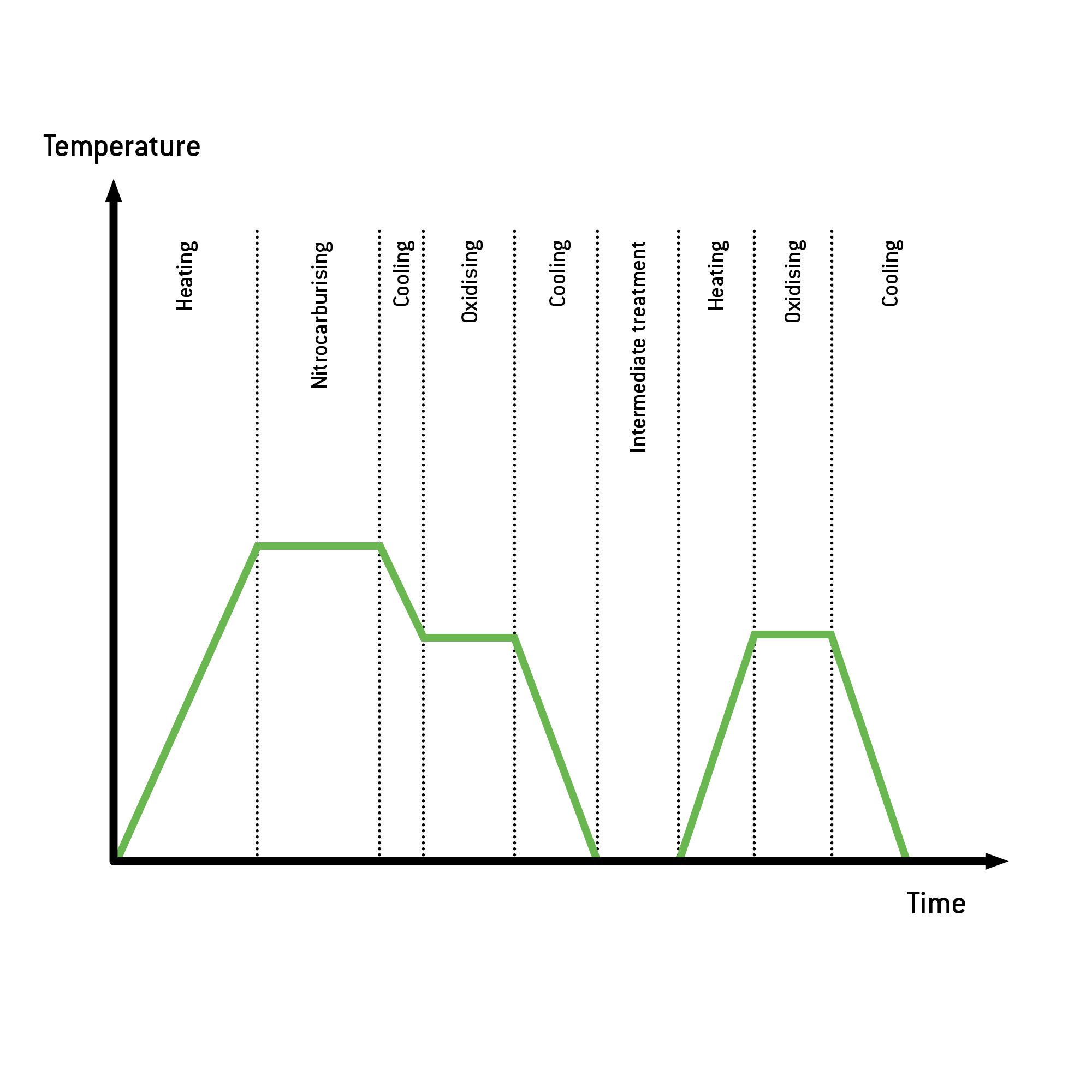
Process flow ALDOX-P
Advantages of the ALDOX process
- Greater surface hardness
- Improved resistance to corrosion
- Increase in wear resistance
- Excellent friction and sliding properties
- High reproducibility
- Attractive dark grey to black colour
- Environmentally friendly method
- Only minimum increase in surface roughness
- Great dimensional stability
- Dimensional changes resulting from manufacturing can be factored in
INFO: Corrosion resistance
The resistance to corrosion depends on various factors, such as the material used, the surface roughness, possible impurities and the geometry of the component. For many materials, ALDOX-S and ALDOX-P exceed the requirements commonly imposed on corrosion resistance. This has been confirmed by a salt mist spray test according to DIN EN ISO 9227 NSS:2017-07.
Areas of application
The ALDOX processes allow for the treatment of a wide range of materials, including non-alloy and low-alloy steels, tool steels, cast materials and sintered iron. The treated workpieces are perfect for use in the automotive industry as well as in areas of mechanical and plant engineering.
Der Beitrag ALDOX – Nitrocarburieren mit Nachoxidation erschien zuerst auf HÄRTHA GROUP.
]]>
The process
Oxidising is a post-treatment which follows nitriding and applies an oxide layer to workpieces, thus creating a noticeable improvement in a wide variety of properties. The process is carried out by introducing oxygen at a temperature up to 570 °C.
Nitriding and nitrocarburising create a thin compound layer of only a few micrometres on the workpiece. During oxidising, the free iron molecules as well as the iron nitrides in this compound layer react with the introduced oxygen to produce stable iron oxide, which then accumulates as a thin oxide layer of no more than 3 µm on the surface of the component. This layer is extremely resistant to chemicals and, together with the compound layer, it gives the workpiece high corrosion resistance and other important properties.
If post-oxidation is planned, it forms the final step of the process, immediately after nitriding. The surface must not be processed further after oxidising, as this would remove the protective layer. The formation of a compound layer during nitriding is a prerequisite for successful, long-lasting oxidation. This is because the hardened compound layer consists mainly of iron nitride, while the underlying diffusion layer contains ferrite, to which the oxide layer adheres much less strongly.
Suitable materials
Since oxidising is a post-treatment of nitrided workpieces, all metals that can be nitrided are suitable for this process. In principle, these include all common cast and sintered materials, as well as non-alloy, low-alloy, and high-alloy steels.
Areas of application
Oxidising is a good alternative to burnishing, and can also be applied to materials that are not suitable for burnishing. Compared with burnishing, the protection against corrosion achieved by oxidising is considerably more effective. Studies have shown that it is comparable to the corrosion protection provided by a hard chrome coating with a thickness of 10 μm.
In addition to improving its mechanical properties, the process also enhances the component surface visually by giving it a colour that ranges between grey and black. The exact shade of the surface depends on the grade of the steel.
Oxidising is especially recommended for nitrided parts made of low-alloy materials where the requirements call for high resistance to wear and corrosion. These components include hydraulic cylinders, transmission spindles, and other moving and friction-loaded components.
The advantages at a glance
As a post-treatment, oxidising offers a wide range of advantages. Especially when combined with nitriding, these have a positive effect on the practical use of the components:
- high level of corrosion protection
- excellent resistance to wear
- improved operating characteristics
- enhanced sliding performance
- visual enhancement thanks to black colouring
Process locations
You will find Härtha in Germany, Italy, and the Netherlands. Refer to our interactive location overview to learn which other processes besides oxidising we offer at locations near you.
Der Beitrag Oxidieren erschien zuerst auf HÄRTHA GROUP.
]]>Egal ob Gasnitrieren oder andere moderne Verfahren: Wir beraten Sie gerne persönlich zur Optimierung Ihrer Bauteile. Ganz nach Bedarf als Einzelteil, in Sondergrößen oder als Serienproduktion. Wir verfügen über mehrere Kammeranlagen und Schachtofenanlagen bis Ø 1.100 x 3.300 mm.
Der Beitrag Gasnitrieren erschien zuerst auf HÄRTHA GROUP.
]]>The process and its advantages
Gas nitriding improves the surfaces of iron-based workpieces that are subjected to high loads. Gas nitriding increases fatigue strength as well as wear resistance and resistance to wear due to scoring. It is carried out at low temperatures around 520°C. This ensures that warping is extremely low. Another version of gas nitriding is gas nitrocarburising, which involves the use of a gas atmosphere which provides carbon.
Gas nitrocarburising creates deeper layers than the Tenifer QPQ process (salt bath nitrocarburising). The treatment times can be extensive in some cases. Components that are sensitive to warping should be pre-treated beforehand by stress-free annealing.
Gas nitriding forms a compound layer on the workpieces that is composed of nitrides. A thin layer improves fatigue resistance. Thick layers improve resistance to wear and corrosion. Dissolved nitrogen atoms and nitride precipitates form the diffusion layer underneath the compound layer. The diffusion layer extends the service life of the components.
NIOX and ALDOX: A special variation of gas nitrocarburising with post-oxidation
If workpieces of premium quality are to undergo gas nitrocarburising and post-oxidation, they can be treated using the NIOX or ALDOX process. The surface receives a refined black look and significantly improved corrosion protection.
Gas nitriding offers a particularly wide range of applications, because different nitriding depths and temperatures can give the workpieces highly diverse properties.
The advantages of gas nitriding at a glance
- Higher bending fatigue resistance and fatigue strength
- Enhanced wear and scoring resistance
- Lower friction coefficient
- Good temperature resistance
- Reliably reproducible results
- Partial hardening possible
- Minimum warping makes reworking the components unnecessary
Surface hardness and nitriding hardness depth of various materials
The table below shows the surface hardnesses that gas nitriding can achieve on various materials.
The following applies:
- The higher the treatment temperature, the lower the intrinsic hardness of the nitrided layer
- The longer the nitriding time, the greater the nitriding hardness depth (NHD).
- The higher the temperature (500°C - 600°C), the deeper the nitrogen diffuses into the material in the same treatment time
- The higher the alloy content of the material, the higher the nitriding hardness of the material. However, the nitrogen’s penetration depth into the material is also decreased.
Areas of application
Gas nitriding is particularly suitable for alloy materials, as they contain nitride-forming elements. Examples include chromium, molybdenum, vanadium, and aluminium. These materials include tool steels (cold worked and hot worked steels as well as sectional steels) and spring steel, which is widely used in the automotive industry. Materials previously hardened or tempered will produce the best results. In contrast, plasma nitriding is more commonly used for stainless steel or high-alloy materials.
Typical components:
- Gearboxes
- Valve components
- Springs
- Gear wheels
- Drive shafts
- Guide rails
- Extruder screws
- Spindles
- Cam discs
- Hydraulic cylinders
- Forging dies
- Bending punches
- Die plates
- Injection moulding tools
- Camshafts
- Crankshafts
- Injection nozzles
Summary: Which materials are suitable?
- Nitriding steels (for high surface hardness alloyed with aluminium, chromium, molybdenum, and vanadium)
- Quenched and tempered steels
- Steel materials
- Cast materials
- Sintered materials
Check list: Ordering gas nitriding
If you would like us to treat your workpieces or materials using gas nitriding, we will gladly advise you on the best procedure. You can use the check list below to make your preparations for the order.
- Which material is to be treated, and what is its condition?
- What is the target hardness (including tolerance range in HV)?
- What is the desired nitriding hardness depth (including tolerance range in mm)?
- If applicable, which areas should be nitrided, and where can the hardness be measured?
- If applicable, how thick should the compound layer be (including tolerance range in μm)?
For gas nitriding to be successful, the surface of the workpiece must additionally meet the following requirements:
- Free of oil, grease, and rust
- Metallically blank
- Neither decarbonised nor oxidised
- No edge layers
- Ideally no surface compaction present, as this hinders the formation of the nitrided layer
Key technical figures:
- Temperature: up to 600 °C
- Size of furnace: Diameter up to 1,100 X 3,300 mm
- Processing time: 12 hours or longer
- Nitriding hardness depth: up to 0.6 mm
Der Beitrag Gasnitrieren erschien zuerst auf HÄRTHA GROUP.
]]>Bauteiloberfläche durch Behandlung in einem Nitrierbad (Salzschmelze) mit anschließender
Oxidation in einem Abschreckbad. Durch Polieren (Strahlen) können die Charakteristika der
Oberfläche weiter verbessert werden.
Beim Salzbadnitrieren wird das Werkstück nur mit Stickstoff angereichert. Das
Salzbadnitrocarburieren (Tenifer-Verfahren) behandelt Werkstücke neben Stickstoff zusätzlich mit
Kohlenstoff. Sie können beide Verfahren bei uns in Auftrag geben.
Egal ob Salzbadnitrieren oder andere State of the Art Verfahren: wir optimieren für Sie auch
anspruchsvollste technische Bauteile. Ganz nach Bedarf als Einzelteil, in Sondergrößen oder als
Serienproduktion.
Der Beitrag Salzbadnitrieren erschien zuerst auf HÄRTHA GROUP.
]]>
The process and its advantages
Salt bath nitriding starts off with a heat treatment of the workpieces at approx. 350°C. This treatment is followed up with the creation of a protective diffusion zone in a 580 °C nitriding bath consisting of a salt melt. During this treatment, both nitrogen and carbon penetrate the surface of the component.
For the desired properties, the chemical composition of the salt melt is controlled accordingly. The treatment can last from a few minutes to several hours, depending on the type of steel. Once the treatment time has elapsed, water, oil, or a polymer is used to quench the workpieces.
Post-treatment in an oxidation bath: Tenifer QPQ process
In the Tenifer QPQ process, workpieces are placed in an oxidation bath after the nitriding bath. The workpieces are then quenched with water. Tenifer creates extremely high corrosion resistance, often surpassing even galvanic coatings. Additionally, the workpieces obtain an elegant blackened surface.
The advantages of salt bath nitriding/salt bath nitrocarburising at a glance
- Improved resistance to wear and corrosion
- Increased fatigue resistance
- Pleasing aesthetics of the components thanks to blackening oxidation
- Minimum warping makes reworking the components unnecessary
- Reliably reproducible results
- Short treatment times
- The nitrided layer is heat-resistant up to 600°C
- Partial hardening possible
- High-alloy and high-chromium steels can also be treated
Areas of application
In general, all grades of steel are suitable for salt bath nitriding. However, certain alloy steels are especially suitable for this process. The process is a particular favourite in mechanical engineering and the automotive industry.
Summary: Typical real-world applications
- Mechanical and apparatus engineering
- Vehicle construction
- Precision engineering
- Automotive industry
Summary: Which materials are suitable?
- Steel materials
- Cast materials
- Sintered materials
- Non-alloy materials
- Low-alloy materials
- Medium-alloy materials
Check list: Ordering salt bath nitriding
If you would like us to treat your workpieces or materials with a salt bath nitriding process, we will gladly advise you on the best procedure. You can use the check list below to make your preparations for the order.
- Which material is to be treated, and what is its condition?
- What is the target hardness (including tolerance range in HV)?
- What is the desired nitriding hardness depth (including tolerance range in mm)?
- If applicable, which areas are to be salt bath nitrided, and where can the hardness be measured?
- If applicable, how thick should the compound layer be (including tolerance range in μm)?
Note:
We use a sample that we take ourselves to measure the thickness of the compound layer and/or the nitriding hardness depth. For measurements that are specifically related to your order, we need you to provide us with a reference component that is intended for the hardening treatment.
Process locations
We offer state-of-the-art salt bath nitriding processes at the following locations: location overview
Der Beitrag Salzbadnitrieren erschien zuerst auf HÄRTHA GROUP.
]]>Egal ob Nitrieren oder andere State-of-the-Art-Verfahren: Wir optimieren Ihre Bauteile als Einzelteil, in Sondergrößen oder als Serienproduktion. Melden Sie sich bei uns für ein persönliches und unverbindliches Beratungsgespräch.
Der Beitrag Nitrieren erschien zuerst auf HÄRTHA GROUP.
]]>The process and its advantages
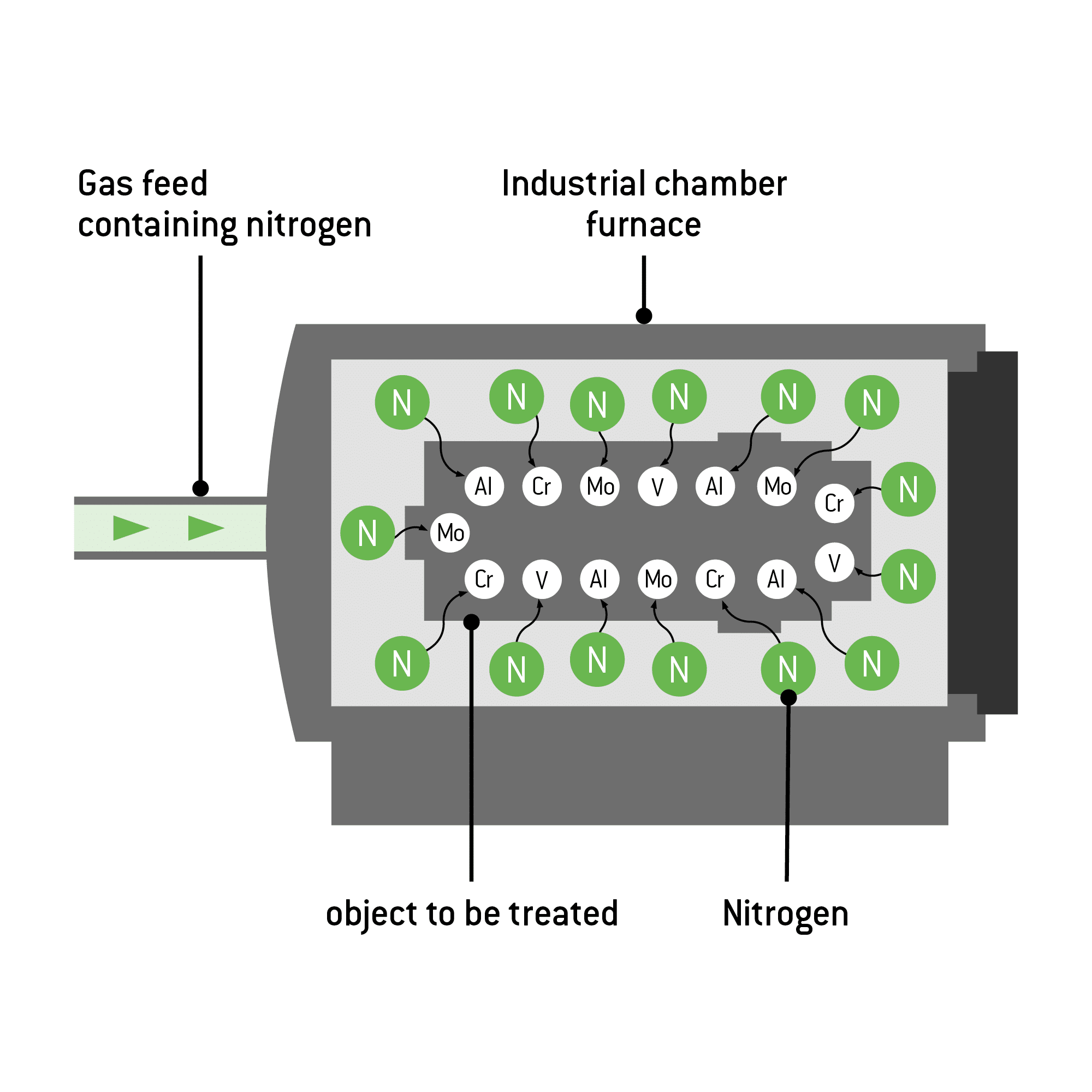
Nitriding (or nitridation) enriches workpieces with nitrogen, causing nitrides to form within them. The nitrogen penetrates the workpiece and forms on its surface a hard compound layer composed of iron nitrides. Located underneath is the diffusion zone. In this zone, nitrogen embeds in the metal matrix of the workpiece, thus increasing its fatigue strength. The highest values can be achieved with nitriding steels.
Nitriding requires temperatures between 500°C and 580°C. Treatment times vary between one and one hundred hours. The thickness of the compound layer and the development of the porous zones depend on the temperature and duration of the treatment. To define the nitriding hardness depth (NHD) of the treatment, the limit hardness is used as a reference. This is 50 HV higher than the core hardness of the workpiece.
The layer’s resistance to corrosion can be further improved by oxidation. If additional coatings (galvanic or chemical) are planned later on, the workpiece can also be nitrided without a compound layer.
The process is different from nitrocarburising, which involves the additional use of carbon.
Graphical representation: Structure and functional principle
The most commonly used nitriding processes are:
Each of these processes also permits partial nitriding. During salt bath nitriding, the workpieces are dipped only partially in the salt bath. The partial treatment during plasma nitriding is accomplished by applying a protective compound or by mechanical means.
The advantages of nitriding at a glance
- Improved hardness of the surface
- Less wear
- High resistance to corrosion
- Lower friction coefficient
- The nitrided layer is heat-resistant up to 600°C
- Partial hardening possible
- Many possible applications, because almost any type of steel can be nitrided. Suited best, however, are alloy steels.
Areas of application: Suitable steels
Almost all cast and sintered materials and grades of steel can be nitrided.
Alloy steels achieve better results than non-alloy steels. With the latter, the surface of the material may become brittle instead of hard. For this reason, low- or non-alloy steels are nitrocarburised instead. This process produces a compound layer that improves protection against wear and corrosion. This compound layer can also be subjected to post-oxidation. That creates another thin oxide layer with a thickness between 1 – 3 µm and provides even greater protection.
In contrast, elements such as molybdenum, titanium, chromium, or aluminium contained in alloy steels produce particularly hard nitrides when reacting with nitrogen. Steels containing a particularly high content of these elements are therefore also referred to as nitriding steels.
These include:
- 1.8519 (31CrMoV9)
- 1.8515 (31CrMo12)
- 1.8550 (34CrAINi7)
- 1.8507 (34CrAlMo5)
You can find a comparison of different methods in the table below.
Process locations
We provide state-of-the-art nitriding processes at the following locations: location overview
Check list: Ordering nitriding
If you would like us to nitride workpieces or materials, we will gladly advise you on the best procedure. You can use the check list below to make your preparations for the order.
- Which material is to be treated, and what is its condition?
- What is the target hardness (including tolerance range in HV)?
- What is the desired nitriding hardness depth (including tolerance range in mm)?
- If applicable, which areas should be nitrided, and where can the hardness be measured?
- If applicable, how thick should the compound layer be (including tolerance range in μm)?
Note: We use a sample that we take ourselves to measure the thickness of the compound layer and/or the nitriding hardness depth. For measurements that are specifically related to your order, we need you to provide us with a reference component that is intended for the hardening treatment.
Der Beitrag Nitrieren erschien zuerst auf HÄRTHA GROUP.
]]>Werkstoffs und erhöht vor allem den Verschleißschutz und die Dauerfestigkeit. Die Oberflächenschicht des Werkstoffs wird dabei mit Stickstoff und Kohlenstoff angereichert. Anders als beim Nitrieren, bei dem nur Stickstoff in die Randschicht eindringt.
Egal ob Nitrocarburieren oder andere State-of-the-Art-Verfahren: wir optimieren für Sie auch
anspruchsvollste technische Bauteile. Ganz nach Bedarf als Einzelteil, in Sondergrößen oder als Serienproduktion.
Der Beitrag Nitrocarburieren erschien zuerst auf HÄRTHA GROUP.
]]>The process and its advantages
During nitrocarburising, both nitrogen and carbon penetrate the surface of a workpiece. The surface hardens, while the core retains its softness. The process also produces a thin compound layer that provides a high degree of protection against corrosion and wear. Moreover, the diffusion layer underneath improves fatigue strength.
While similar in name, nitrocarburising and carbonitriding are two completely different processes. Carbonitriding is classified as case hardening, because it does not create a compound layer and only small amounts of nitrogen are used.
Three different types of nitrocarburising
Nitrocarburising can be carried out using different media. Gas is used for gas nitrocarburising, a salt bath for salt bath nitrocarburising and, finally, plasma for plasma nitrocarburising.
Gas nitrocarburising
Good candidates for gas nitrocarburising are low- and non-alloy steel whose wear and corrosion resistance needs to be improved. This process uses a gas mixture such as ammonium and carbon dioxide, which releases both nitrogen and carbon. The treatment temperature is 500°C to 600°C. If the workpiece is quenched in an oxidising atmosphere, corrosion protection can be improved even further.
Salt bath nitrocarburising
Cast iron, as well as low-, medium-, and high-alloy steel, can be subjected to salt bath nitrocarburising, to achieve exceptionally high resistance to wear and corrosion. For this, the workpieces are dipped in a salt melt at temperatures between 550°C and 630°C. This is a blend of alkaline cyanates and alkaline carbonates. Here too, if the workpiece is quenched in an oxidising atmosphere during a post-treatment, corrosion protection can be improved further.
Plasma nitrocarburising
High-alloy steel is suitable for plasma nitrocarburising. If its chromium content is less than 13 per cent, the steel can be subjected to both gas nitrocarburising and plasma nitrocarburising. The workpieces are enveloped by ionised gas at temperatures between 480 and 580°C. No passivation layer is formed. Warping is low, and rework is usually unnecessary.
Nitriding vs. nitrocarburising
Both nitriding and nitrocarburising improve the wear resistance of a component. However, nitrocarburising is capable of achieving substantially greater surface hardness. This makes the process better suited for optimising corrosion protection and vibration resistance. Nitriding, on the other hand, works well in enhancing the wear resistance, as well as sliding and emergency running properties.
Pros and cons of nitriding
- Improved wear protection (abrasive, adhesive, and corrosive)
- Option to precision-harden the edge zones
- Plasma nitriding is the most environmentally friendly process (toxic gases not used)
- Partial hardening possible
Pros and cons of nitrocarburising
- More cost-efficient than carburising and other surface hardening processes
- Improved protection against wear and corrosion
- Optimised sliding properties
- Improved emergency running properties
- Minimum warping thanks to low process temperatures – no need for rework
- Also suitable for treatment of bulk material
Areas of application
Nitrocarburising and nitriding are processes that can be used for the same materials. This also includes non-alloy steel. Because it is exceptionally cost-efficient, the process is used, for instance, instead of classic hard coatings for punching tools.
Typical components
- Gate valves
- Ball seats and ball heads
- Valve shafts
- Control valves
- Impeller housings
- Pump cases
- Pistons and cylinders
- combine harvester separators, crop transfer stations, and crop cutting units
- Oil pump gears for diesel engines
- Gear wheels
- Crankshafts and cam shafts
- Press jaws and cutting dies
- Cam drums
- Extruder and injection moulding machines
- Press drills, enclosures, and press mould components
- Guide tracks for automatic small arms
Surface hardness and nitriding hardness depth of various materials
The table below tells you the surface hardnesses that nitrocarburising can achieve on various materials.
The following applies:
- The higher the treatment temperature, the lower the intrinsic hardness of the nitrided layer.
- The longer the nitriding time, the greater the nitriding hardness depth (NHD).
- The higher the temperature (480°C - 630°C), the deeper the nitrogen diffuses into the material in the same treatment time.
- The higher the alloy content of the material, the higher the nitriding hardness of the material. However, the nitrogen’s penetration depth into the material is also decreased.
Check list: Ordering nitrocarburising
If you would like us to nitrocarburise your workpieces or materials, we will gladly advise you on the best procedure. You can use the check list below to make your preparations for the order.
- Which material is to be treated, and what is its condition?
- What is the target hardness (including tolerance range in HV)?
- What is the desired nitriding hardness depth (including tolerance range in mm)?
- If applicable, which areas should be nitrided, and where can the hardness be measured?
- If applicable, how thick should the compound layer be (including tolerance range in μm)?
Note:
We use a sample that we take ourselves to measure the thickness of the compound layer and/or the nitriding hardness depth. For measurements that are specifically related to your order, we need you to provide us with a reference component that is intended for the hardening treatment.
Der Beitrag Nitrocarburieren erschien zuerst auf HÄRTHA GROUP.
]]>Gasnitrocarburieren. Es verleiht Werkstücken eine extra hohe Korrosionsbeständigkeit und eine edle
anthrazitfarbene bis schwarze Oberfläche. Durch das Verfahren wird eine dünne Oxidschicht
aufgetragen. Damit diese sich ausreichend bilden kann, müssen die Werkstoffe zwingend durch
Nitrieren oder Nitrocarburieren vorbehandelt werden
Egal ob NIOX-Verfahren oder andere State of the Art Prozesse: wir optimieren für Sie auch
anspruchsvollste technische Bauteile. Ganz nach Bedarf als Einzelteil, in Sondergrößen oder als
Serienproduktion.
Der Beitrag NIOX – Nitrocarburieren mit Nachoxidation erschien zuerst auf HÄRTHA GROUP.
]]>The process and its advantages
In the NIOX process, the nitrocarburising is followed up by a post-oxidation of the workpieces. In this step, the compound layer produced in the preceding step is partially converted into an oxide layer by dwelling and cooling in an oxidising environment. This layer further increases the corrosion resistance of the workpiece surface.
Beim Aldox-Verfahren werden im Vergleich zum NIOX-Verfahren eine Zwischenbehandlung und ein zusätzlicher Oxidationsprozess durchgeführt. So wird der Korrosionsschutz noch weiter erhöht.
Materials made of low-alloy steel benefit especially from a NIOX or ALDOX treatment. As components made of these materials are commonly exposed to great stress (gear spindles, hydraulic cylinders, etc.), they require particularly strong protection against wear and corrosion.
The advantages of the NIOX process at a glance
- Highest corrosion protection out of all the nitriding processes
- Greater wear resistance
- Improved fatigue strength
- Enhanced resistance to tribological and dynamic mechanical stresses
- Optimised sliding characteristics
- Reduced cold weld build-ups
- Resistant to fluctuating temperatures down to the cryogenic range
- Pleasing aesthetics
- Also suitable for treatment of bulk material
Additional advantage of the ALDOX process
The ALDOX process offers even greater corrosion protection, but is also more costly than the NIOX process.
Areas of application
For any application where coatings are used (galvanic, chromated, phosphated, burnished, ...)
nitrocarburising and oxidation offer an attractive and high-performance alternative.
Typical components
- Hydraulic components
- Hydraulic cylinders and pistons
- Screw conveyors
- Pump wheels and enclosures
- Tools and tool holders
- Valves
- Nozzles
- Non-return valves
- Gear wheels and chain wheels
- Moulds, forming tools, dies, and die plates
- Engine parts
- Cam shafts and crankshafts
- Gear parts
- Spindles
- Axles
- Shafts
- Couplings
Der Beitrag NIOX – Nitrocarburieren mit Nachoxidation erschien zuerst auf HÄRTHA GROUP.
]]>in Sondergrößen oder als Serienproduktion.
Der Beitrag Plasmanitrieren erschien zuerst auf HÄRTHA GROUP.
]]>The process and its advantages
Plasma nitriding is a heat treatment process in which the edge layer of a workpiece is converted chemically. During this process, nitrogen penetrates the material and forms nitrides. Plasma nitriding and plasma nitrocarburising deliver reliably reproducible outcomes and are superior to other nitriding processes - especially in terms of environmental protection and energy consumption. The process requires no toxic gases, and the energy consumption is reduced substantially.
This is partially because the hardening process is carried out at low temperatures between 350 and 600°C. Warping of the workpieces is also reduced to a minimum, eliminating the need for costly rework, and thus delivering additional cost savings.
Furthermore, plasma nitriding considerably improves a number of workpiece properties, such as service life, corrosion protection, and resistance to fatigue and wear. A partial treatment is also unproblematic. The process is also suitable for subsequent physical and chemical vapour deposition with individual hardness properties, because if necessary a diffusion layer can be created without a compound layer on the surface of the workpiece.
The process is also known by the names of ionitriding, pulse plasma nitriding, cold nitriding, or plasma hardening.

INFO: HLX-1 – process for special surface protection
HLX-1 is defined as a diffusion process that specifically treats the surfaces of components and tools. HLX-1 creates a protective layer that is ideally suited for structured and polished surfaces. In addition, warping is extremely low.
The advantages of plasma nitriding at a glance
- Improved protection against wear and corrosion
- Excellent vibration resistance
- Fewer brittle and porous layers compared with salt bath nitriding and gas nitriding
- Adaptable layer structure
- Minimum warping thanks to low process temperature, so reworking of components is unnecessary
- Partial hardening possible by applying a protective compound or using mechanical means
- No post-cleaning necessary, because the components receive final cleaning and surface activation in the plasma
- Short treatment times compared with gas nitriding
- Alloy steels and stainless steel can be treated with good results
The physics behind the process
Plasma nitriding takes place in a vacuum. An electric field is applied during the process. The workpieces act as the cathode, and the furnace wall is the anode. The added mixture of gas is ionised by the electric field and it envelops the workpieces. The process creates nitrogen-rich nitrides, which disintegrate and enrich the surface with nitrogen.
The surface is activated, and the workpieces are heated. On steels such as stainless steel, the passive layers detach. Generally speaking, the surface receives fine cleaning, because the process sputters off impurity atoms.
The treatment temperature depends on the material type and the desired nitriding hardness depth. The subsequent dwell time ranges between 12 and 50 hours. To equalise the pressure when the treatment is complete, the furnace is flooded with gas. The workpieces cool down.

Areas of application
Plasma nitriding ranks among the most flexible and best nitriding processes. It is generally suited for any type of iron material, but the benefits of the treatment vary with the material.
- Construction steel: greater protection against wear and corrosion
- Sintered materials: Operating characteristics and wear protection are improved despite porosity
- Alloy steels (with a high chromium and aluminium content): particularly improves components subjected to great strain
- Stainless steel: Wear protection thanks to standard processes with maximum hardness and nitriding hardness depth. Alternative options are long-term, low-temperature processes for high corrosion protection with great surface hardness
Typical components
- Gear shafts, crankshafts and cam shafts
- Cam followers
- Valve components
- Extruder screws
- Die cast tools
- Forging dies
- Tools for cold forming
- Injection nozzles
- Plastic injection moulding tools
- Long shafts
- Axles
- Couplings and engine parts
The nitrided layer and its properties
On the surface, the nitrided layer consists of the compound layer. It is composed of iron nitrides and is more compact and less porous compared with gas nitriding. This layer is followed by the diffusion zone, which is composed of the material and the precipitated nitrides.
If nitriding steel or high-alloy steel with many nitride-forming elements is used, the surface hardness that can be attained also increases accordingly (up to 800–1,200 HV compared to 250-300 HV for non-alloy and 600-700 HV for low-alloy steel). The edge distance having a core hardness of +50 HV is considered to be the characteristic value of the nitriding hardness depth (NHD). This is up to 0.6 mm for non-alloy and low-alloy steels and up to 0.15 mm for high-alloy steels and stainless steel. Influencing factors are the steel used, along with the duration and the temperature of the treatment.
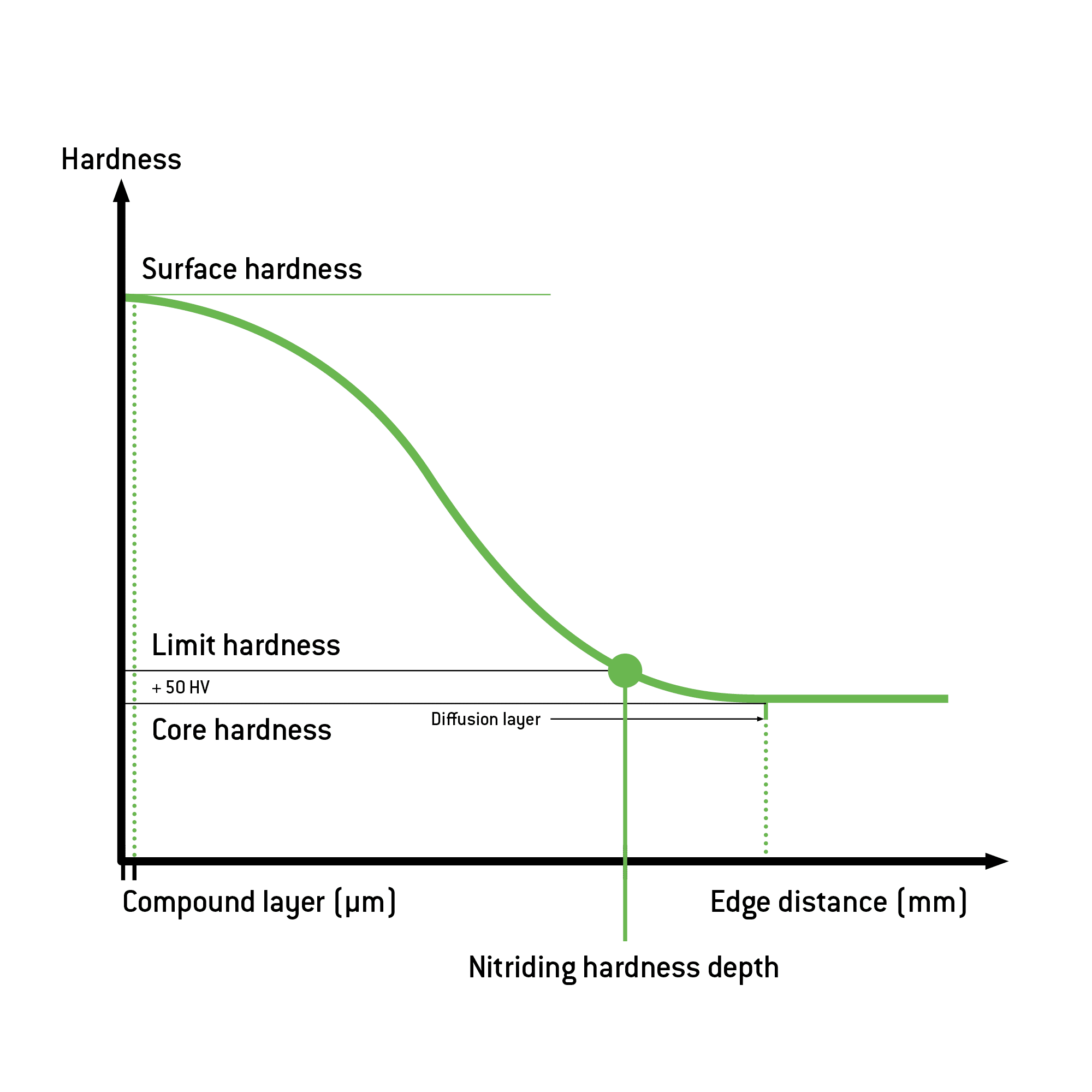
There are variations and enhancements of plasma nitriding, and their use depends on the requirements:
- Plasma nitrocarburising: for particularly thick compound layers
- Post-oxidation: further increases corrosion protection on low- and medium-alloy materials
Nitridable steels and treatment outcomes after plasma nitriding
The following results illustrate the effect of plasma nitriding on frequently used materials. Standard and long-term treatments were used. A higher or lower nitriding hardness depth (NHD) and compound layer thickness (CLT) can be achieved by special treatments.
Der Beitrag Plasmanitrieren erschien zuerst auf HÄRTHA GROUP.
]]>